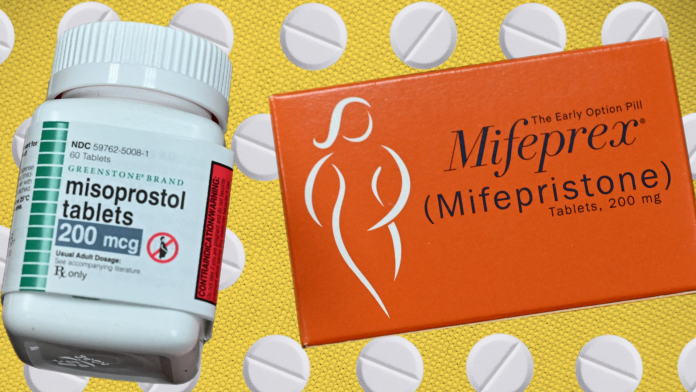
The United States has approved the sale of mifepristone, an abortion pill, at pharmacies.
Mifepristone is taken in combination with a second drug called misoprostol to end an intrauterine pregnancy at ten weeks gestation (70 days or less since the first day of a patient’s last menstrual period).
The US Food and Drug Administration (FDA) first approved the drug in 2000 but patients could only obtain it in person from a health provider.
On Tuesday, the FDA updated its website with the new requirements for the drug, saying it “can be dispensed by certified pharmacies or by or under the supervision of a certified prescriber”.
A prescription is, however, still required under the new rule.
The agency said the decision was reached after conducting a review of the risk evaluation and mitigation strategy (REMS).
“The FDA determined that the available data and information support modification of the REMS to reduce burden on the health care delivery system and to ensure the benefits of the product outweigh the risks,” the agency said.
US pharmacies can now apply for certification to distribute Mifepristone, which will allow them to directly sell to customers who have a prescription from a certified prescriber.
The move is said to be an effort by President Joe Biden’s administration to protect abortion rights following the supreme court’s decision to overturn the right to abortion, with several states banning or restricting access to the service.
Women in states where abortion has been banned will likely need to travel to other states to obtain medication for abortion.